dISCOVERY OF THE NEW fusion chromo device
by pHILIPPE pecile
This seminar introduces the participants to the new Fusion Product custom made
Hybrid session on 8 October 2022 at 10:00 and 16:00 CET
Workshop
The main objective of this workshop is to introduce the new product Fusion
8 October 2022
Days
Hours
Minutes
Seconds
SPEAKERs
Philippe Pecile
Chromoterapist and diver. He will introduce the new product line Fusion developed on his demand
Looking for targeted customer ?
Meet decision makers in the pharmaceutical and cosmetic industry
Show off your solutions to key influencers
Gain visibility by editorial relay on labhnn communications
Stay up to date
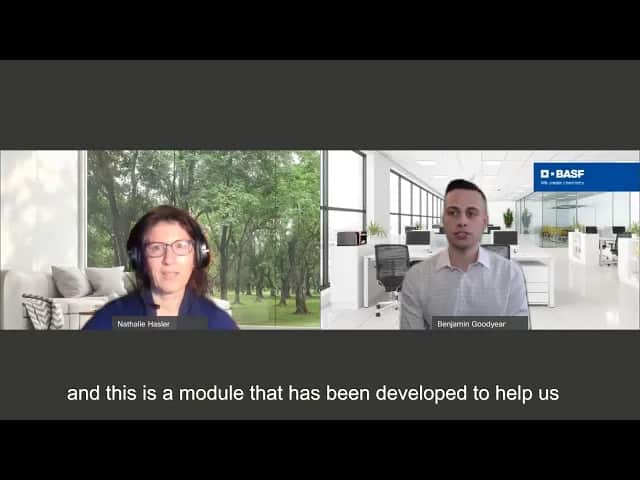
Labhnn Hybrid Workshop on 6 April 2021 – BASF Platin Sponsor
You never formulated a topical medicated product, and you wish to speed up its development. How to start?
March 19, 2021
No Comments
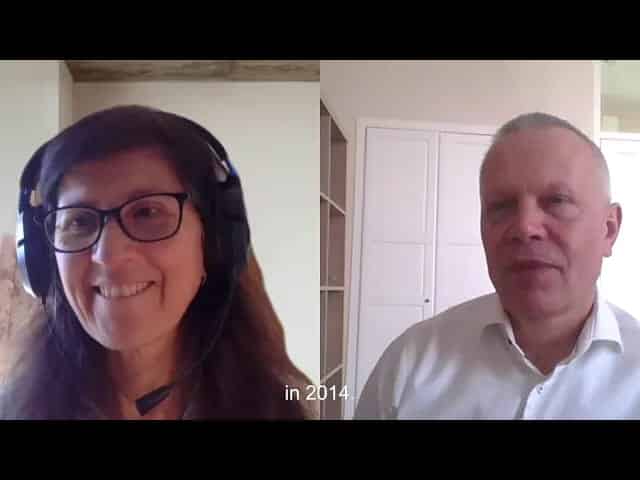
Include early clinical results in the development of your topical dermal product
How to include early clinical results in the development of your topical dermal product?
RiverD, has a solution. Watch our interview
March 17, 2021
No Comments

Molecular therapeutics delivery group
Introduction of Dr. Maria Lapteva from University of Geneva Meet Dr. Maria Lapteva, senior scientist at Prof. Kalia’s lab in the Pharmaceutical School of the
March 8, 2021
No Comments
